The abundance of the nitrogen-14 isotope is 996 percent and the abundance of the nitrogen-15 isotope is 04 percent. 24Mg 7870 25Mg 1013 and 26Mg 117.

Handout 03 Average Atomic Mass
Lithium has two naturally occurring isotopes.
. Add the similar terms in the equation to get the final answer. But from the question we are not given these values which means we must think of a way of finding them. Europium-151 has an abundance of 4803 and Europium-153 has an abundance of 5197.
The three Potassium isotopes have atomic masses and percent compositions of 39 amu 648 40 amu 186 and 41 amu 166. A Hydrogen perchlorate HOCl b Ammonium sulfate NH 4 2 SO 4 c Urea NH 2 2 CO d. The answer 12011 amu is the same value found for.
Naturally occurring europium Eu consists of two isotopes was a mass of 151 and 153. Carbon 6 C 12011 isotope abundance mass amu. To find the average atomic mass for Carbon.
What is the atomic mass of europium. Worksheet to help students calculate RAM from relative abundances of isotopes. Using the following data calculate the average atomic mass of magnesium give your answer to the nearest 01 u.
Calculate the abundances if the atomic weight is 4268Â Since does not exist you cannot search for exact isotope weights. Isotope Practice Worksheet 1. Divide the mass of each isotope beans peas and corn by the number of each isotope to get the average mass of each isotope.
The table below shows the exact mass of each isotope isotopic mass and the percent abundance sometimes called fractional abundance for the primary isotopes of Carbon. Finally solving for x we get x076. It has two isotopes.
Calculate the relative atomic mass of strontium. The percent abundance of these isotopes is as follows. Antimony has two naturally occurring isotopes.
Strontium consists of four isotopes with masses of 84 abundance 050 86 abundance of 99. Determine both the percentage abundance and the relative isotopic mass of the second isotope. Calculating percent abundance of isotopes worksheet.
The mass of antimony-121 is 120904 amu and the mass of antimony-123 is 122904 amu. Consider that an element has two isotopes. Average Atomic Mass 120000 9890 130033 00110 12011 amu.
Here are three isotopes of an element. How to Calculate Percent Abundance. Calculate the percentage abundance of each isotope.
What is the average atomic mass of europium. 65Cu 649278 amu 7 Magnesium consists of three naturally occurring isotopes. Calculate the relative abundance of the unknown isotope.
Calculating percent abundance of isotopes worksheet Author. Find the percent abundance. The average atomic mass between these two isotopes is 63546 amu.
Atomic mass mass 1 1 mass 2 2. Given the atomic weight of copper of 63546 what is the percent abundance of each. 1 Three isotopes of Silicon occur in nature.
Ad Download over 20000 K-8 worksheets covering math reading social studies and more. When two atoms of the same element have a different number of neutrons and they Created Date. Now we will use the algebra to find the value of x.
Find the percentage. 12 25Mg Percent abundance. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance.
Calculating atomic mass worksheet. The other isotope 65Cu has an abundance of 3091. 7 Calculate the relative molecular mass of each of the following compounds.
Naturally occurring europium Eu consists of two isotopes was a mass of 151 and 153. Silicon-28 9223 2797693 amu Silicon-29 468 2897649 amu Silicon-30 309. Atomic Mass of each isotope and abundance of each isotope 3.
Given below is a problem with its detailed solution explaining how to find the same. Structured to start by thinking of balls in a box through to simple calculation for copper. 3 1 9 6.
Using the average mass from the periodic table calculate the abundance of each isotope. Chlorine consists of two isotopes with masses of 35 abundance 75 and 37 abundance of 25. Europium-151 has an abundance of 4803 and Europium-153 has an abundance of 5197.
Calculate the average atomic mass of bromine showing all work. If the atomic masses of these isotopes are given calculating the percentage abundance of each of the isotopes is possible. Using these data calculate the approximate atomic mass of lead.
The relative abundances of these four isotopes are 14 241 221 and 524 respectively. Isotope Worksheet CH30S 1. Divide the number of each isotope the total number of particles and multiply by 100 to get the percent abundance of each isotope.
Chlorine has two stable isotopes 3 5 C l and 3 7 C l with atomic masses 349689 u and 369659 u respectively. Abundance is defined as the amount of isotope contained in its parent element. 6 12C 6 13C.
The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l 0. Then like terms is combined given by. The four isotopes of lead are shown below each with its percent by mass abundance and the composition of its nucleus.
How to Calculate Percent Abundance. 79Br of I r 7892 and percentage abundance 5054 and 81Br. The value of atomic mass of copper is 63546.
Calculate the actual atomic mass of 65Cu. Firstly property called distributive is used. Naturally occurring boron B consists of two isotopes with a mass of 10 and 11.
Silver-107 has a mass of 10690509 amu and silver-109 has a mass of 10890476 amu. In this worksheet we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Isotopes - relative abundance calculations structured worksheet.
12 26Mg Percent abundance. Divide the percent abundance from Step 4 by 100 to get the relative abundance. This problem demonstrates finding the percent abundance of isotopes with known average atomic mass.
In this worksheet we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. To calculate percentage abundance we must first know the fractional abundance of each isotope. Calculating Relative Abundance in Mass Spectroscopy If a mass spectrum of the element was given the relative percentage isotope abundances are usually presented as a vertical bar graph.
Calculating Average Atomic Mass Worksheet. Average Atomic Mass Worksheet. Therefore use 42 and 44 in solving the problem.
I find this approach helps students to understand the principle of a weighted average. From these data calculate the. 12 24Mg Percent abundance.
65Cu 305475 Calculation of percent abundance of isotopes is usually done in chemistry worksheets. Copper is made up of two two Cu-63 629296 AMU and CU-65 649278 AMU.

Isotopic Abundance Practice Problems
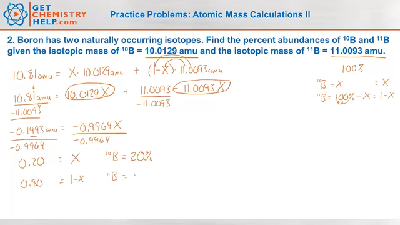
Chemistry Practice Problems Atomic Mass Calculations Ii Get Chemistry Help
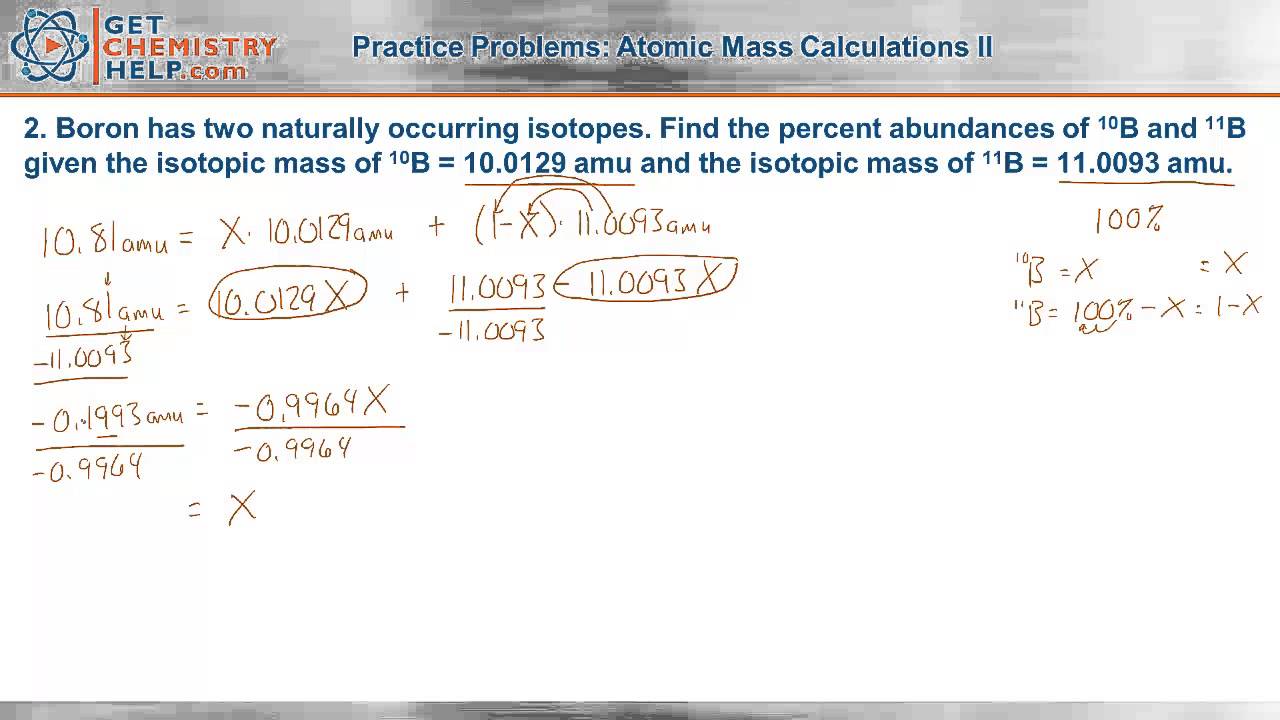
Chemistry Practice Problems Atomic Mass Calculations Ii Youtube

Find The Average Atomic Mass Example Magnesium Chemistry Class School Help Chemistry

Calculating The Average Atomic Mass For Neon Example Youtube

How To Calculate Percentage Abundance Using Atomic And Isotopic Masses

Calculating Average Atomic Mass Chemistry Lessons Teaching Chemistry Chemistry Classroom

Average Atomic Mass Practice Problems Youtube

Isotopes Relative Abundance Calculations Structured Worksheet Teaching Resources

The Atom Chemistry Help Chemistry Notes Atom
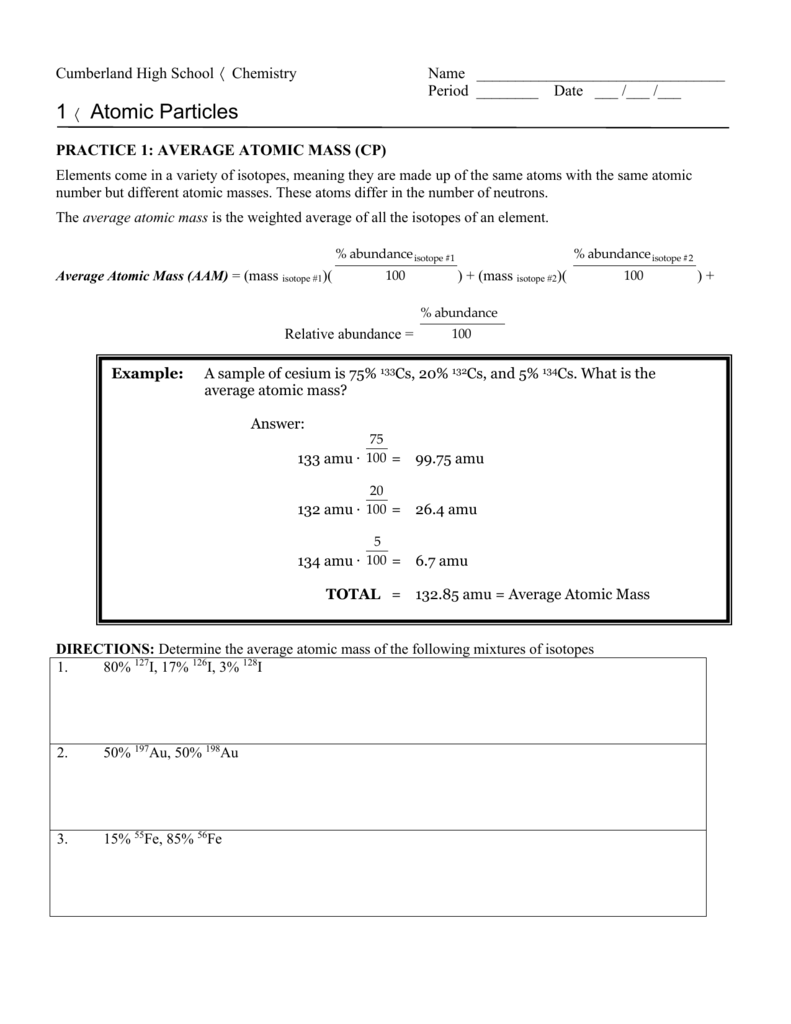
Practice 1 Average Atomic Mass

Solving For Percent Abundance With Isotopes Chemistry Sample Problem Youtube

Solving For Percent Abundance With Isotopes Chemistry Sample Problem Youtube

Solve For Isotopic Abundance Chemistnate

Chemistry How To Calculate Atomic Mass Of An Element Isotopes Isotope Notation Atomic Mass Unit Teaching Chemistry Chemistry Lessons Relative Atomic Mass
Tidak ada komentar:
Posting Komentar